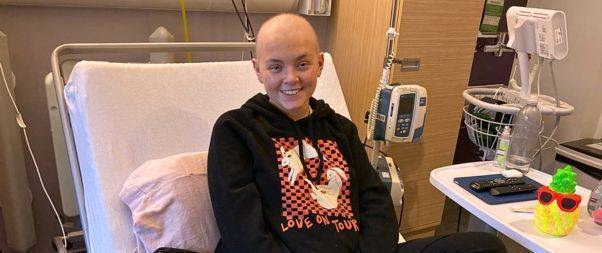
The 19-year-old “cancer warrior” Kira Noble has achieved remission following months of chemo-immunotherapy.
Kira began the innovative treatment for Neuroblastoma in January, along with physical therapy.
This Edinburgh teenager, who is battling a rare form of cancer, is now living her life to the fullest.
She has also advocated for more compassionate treatment options for children coping with the illness.
Let’s discuss more about her illness in detail:
What is Neuroblastoma?
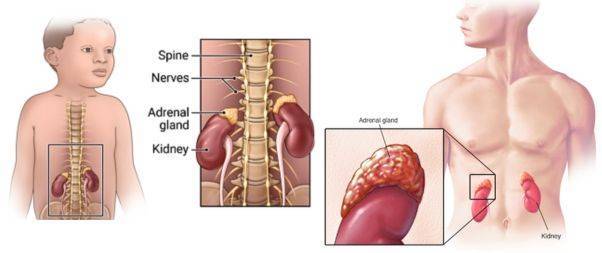 Neuroblastoma, a type of cancer that develops from immature nerve cells called neuroblasts.
Neuroblastoma, a type of cancer that develops from immature nerve cells called neuroblasts.
It is most commonly found in infants and young children, although it can occasionally occur in older children and adults.
Neuroblastoma is a rare cancer, accounting for about 6% of all cancers in children.
It is typically originates in the adrenal glands, which are located on top of the kidneys and produce important hormones.
However, it can also develop in other parts of the body where nerve tissue is present, such as the neck, chest, or spine.
The cause of Neuroblastoma is not well understood. While there are a few rare instances of multiple children within the same family being affected, it is generally not considered a hereditary condition.
Common Signs and Symptoms of Neuroblastoma

Symptoms of neuroblastoma can result from the tumor pressing on nearby tissues as it grows or the cancer spreading to the bone. While these symptoms may be caused by neuroblastoma, they could also indicate other conditions.
Consult your child’s doctor if your child experiences any of the following:
- A lump in the abdomen, neck, or chest.
- Bone pain.
- Swollen stomach and difficulty breathing (in infants).
- Protruding eyes.
- Dark circles around the eyes (“black eyes”).
- Painless, bluish lumps under the skin (in infants).
- Weakness or paralysis (inability to move a body part).
Less frequent signs and symptoms of neuroblastoma include:
- Fever.
- Shortness of breath.
- Fatigue.
- Easy bruising or bleeding.
- Petechiae (flat, pinpoint spots under the skin due to bleeding).
- High blood pressure.
- Severe watery diarrhea.
- Horner syndrome (droopy eyelid, smaller pupil, and reduced sweating on one side of the face).
- Jerky muscle movements.
- Uncontrolled eye movements.
How to Diagnose Neuroblastoma?

If your child is suspected to have neuroblastoma, several tests may be performed to confirm the diagnosis. These tests may include:
- Urine test: To check for specific chemicals produced by neuroblastoma cells that are found in urine.
- Scans: Ultrasound, CT, and MRI scans of various body parts may be performed to examine these areas in detail.
- MIBG scan: This test involves injecting a substance that is absorbed by neuroblastoma cells.
- Biopsy: A sample of tumor tissue is removed and examined under a microscope to identify the type of cancer. The sample is typically removed under general anesthesia using a special needle.
- Bone marrow biopsies: To check for the presence of cancer cells in the bone marrow.
After these tests are completed, a diagnosis of neuroblastoma can usually be confirmed, and the stage of the cancer can be determined.
Stages of Neuroblastoma
For neuroblastoma, the stage affects the classification of the cancer as low risk, intermediate risk, or high risk, which in turn influences the treatment plan.
The staging system includes:
- Stage L1: The cancer is localized to one area, has not spread, and can be surgically removed.
- Stage L2: The cancer is localized to one area and has not spread, but cannot be safely removed through surgery.
- Stage M: The cancer has metastasized to other parts of the body.
- Stage Ms: The cancer has spread to the skin, liver, or bone marrow in children under 18 months of age.
Determining the stage of your child’s neuroblastoma helps doctors decide on the most appropriate treatment plan.
Treatment and Prognosis
In some cases, babies and infants under 18 months old with stage L1 or Ms neuroblastoma and no symptoms may not require treatment, as cancer can occasionally resolve on its own.
Main treatments for neuroblastoma include:
- Surgery to remove the cancer – this may sometimes be the only required treatment.
- Chemotherapy – this can either be the sole treatment or used to shrink the cancer before surgery.
- Radiotherapy – this may be used post-surgery to destroy any remaining cancer cells in the affected area.
- High-dose chemotherapy followed by a stem cell transplant – your child’s stem cells are collected, frozen, and stored before intensive chemotherapy, and then reintroduced afterward.
- Immunotherapy – a medication that directly targets neuroblastoma cells, although not yet routinely used.
Dinutuximab beta
It is a new medication that can be employed when neuroblastoma is likely to recur after conventional treatment.
It is available on the NHS for children who have partially or fully responded to high-dose chemotherapy followed by a stem cell transplant and have not already received immunotherapy.
Your child’s doctor can determine if dinutuximab beta is a suitable treatment option.
This medication targets and kills cancer cells and is administered through an infusion pump into a vein. A treatment course lasts 35 days, during which your child may need to stay in the hospital.
Dinutuximab beta can cause nerve pain, particularly at the beginning of the course. Consequently, it is administered in combination with a strong painkiller like morphine, which may make your child drowsy.
Prognosis
The prognosis for neuroblastoma varies significantly, with younger children and those whose cancer has not spread generally having a better outlook. Your child’s doctors can provide more specific information.
Nearly half of all neuroblastomas are a type that can recur despite intensive treatment, often necessitating further therapy.
Clinical Trials
Upon your child’s neuroblastoma diagnosis, you may be invited to participate in a clinical trial. Clinical trials aim to evaluate the effectiveness of various treatments.
If you are interested, consult your child’s doctors about any trials they may be eligible to join. Additionally, you can search the database of clinical trials for neuroblastoma to learn about ongoing research in your area.
The WHO International Clinical Trials Registry Platform (ICTRP) website provides access to clinical trials in countries all around the world.
Battle of Teen ‘Cancer Warrior’ Kira Noble

Kira Noble was diagnosed with aggressive Neuroblastoma at age 11, and has undergone several years of grueling cancer treatments while spending extended periods in the hospital.
Despite battling her 6th relapse after 20 rounds of Chemotherapy, Kira now leads a more normal teenage life, thanks to the wonder drug Lorlatinib. Known as “Kira the Machine” for her resilience, she was informed in 2019 that her illness was incurable but treatable.
Kira’s family reports significant improvements since she began taking the experimental drug two and a half years ago when it was still undergoing clinical trials.
After recovering from Covid-19, Kira’s mother expressed her joy at seeing her daughter regain control of her life, following years of harsh treatments.
Kira, a passionate advocate for childhood cancer research, emphasizes the importance of better, kinder treatments for children with cancer.
Currently working in retail at St James Quarter, Kira enjoys spending time with friends and is interested in health and beauty.
She deferred her university admission to next year saying “I also had a University placement to study Paediatric Nursing on the horizon but had to pend that whilst I underwent my chemoimmunotherapy treatment. I will reapply when the time is right and when my health is back on par.”
Kira’s mother highlighted the need for gentler treatments for children, as Kira underwent 20 rounds of chemotherapy over 4 years, causing significant harm to her developing body.
She described the difference in her daughter’s quality of life while on Lorlatinib as incredible.

In a social media post, her mother added: “We are so grateful that the effect of chemo-immunotherapy eradicated cancer but we remain mindful of how pernicious this deceitful disease is.
“Kira will remain on her ALK inhibitor, Lorlatinib, in the hope that it maintains her disease for a longer period of time.
“Hoping, praying, and envisioning positive outcomes for this treatment.”