“Renowned for his sharp wit and surreal humor, SEAN LOCK was an adored comedian who entertained countless fans worldwide.”
A well-known British comedian, actor, and writer passed away on August 16, 2021, at the age of 58 had been battling cancer for some time before his death.
Lock’s agent confirmed in a statement that he “died at home from cancer, surrounded by his family.” The statement also mentioned that Lock had been “bravely” undergoing treatment for several years before his death.
What illness did Sean Lock have?

In 2021, Sean Lock passed away after a long battle with “lung cancer” that had been diagnosed years earlier: his buddy Bill Bailey has affirmed.
The news was confirmed by his management company, Off The Kerb Productions, who stated that Lock was surrounded by his family when he died at home.
He’s amazingly courageous and tough individual who was mentally strong throughout the whole time. I’m just grateful for the time we have had together.” said Bill Bailey.
Lock was not only a talented comedian but also a devoted husband and father of three children, who will undoubtedly miss him dearly.
His boundless creativity, lightning wit, and unique voice in British comedy made him one of Britain’s finest comedians, and his absence in the entertainment industry is felt by many.
The loss of Sean Lock is a reminder of the devastating impact of cancer on individuals and their loved ones, highlighting the importance of early detection, regular health screenings, and timely medical intervention.
What is Lung Cancer?

Primary lung cancer refers to cancer that starts in the lung tissue, windpipe (trachea), or the main airway (bronchus). This type of cancer can present itself in different forms, with non-small cell lung cancer being the most prevalent. Treatment options depend on the type of cancer that is diagnosed.
In contrast, secondary lung cancer, also known as metastatic lung cancer, occurs when cancer from another part of the body spreads to the lungs. The treatment approach for secondary lung cancer may differ from primary lung cancer and depends on various factors, such as the type and stage of cancer and the patient’s overall health.
Warning Signs

In its early stages, lung cancer may not always exhibit symptoms. However, recognizing the signs and symptoms of lung cancer and seeking medical attention promptly can improve treatment outcomes.
It’s essential to note that many of the symptoms associated with lung cancer can also be caused by other medical conditions.
Some of the most common symptoms of lung cancer include:
- Development of a new cough or persistent coughing
- Shortness of breath or wheezing during routine activities
- Coughing up sputum (phlegm) with blood in it
- Pain or discomfort in the chest or shoulder
- Repeated chest infections or infections that don’t improve with treatment
- Loss of appetite
- Fatigue or constant tiredness
- Unexplained weight loss
If you experience any of these symptoms, it’s advisable to get them checked out by a medical professional as soon as possible. Early detection and treatment of lung cancer can improve survival rates and quality of life.
Lung changes that show on an x-ray

Occasionally, a chest X-ray may reveal unusual changes that require further investigation. In some cases, your doctor may have ordered the X-ray for reasons unrelated to any suspected lung conditions, and you may not exhibit any symptoms.
It’s important to note that changes on a chest X-ray do not always indicate lung cancer. Other factors, such as infections, may cause these changes. However, your doctor may recommend additional tests to examine the changes in further detail to determine their underlying cause.
Hormone related symptoms
While rare, some types of lung cancer cells can produce hormones that enter the bloodstream and cause symptoms unrelated to lung cancer, known as paraneoplastic syndrome. Small cell lung cancer is more likely to cause these symptoms.
Symptoms related to these hormones may include:
- Nausea and vomiting
- Headaches
- Confusion or cognitive impairment
- Weakness or fatigue
- Irritability or restlessness
- Muscle weakness, spasms, cramps, or aches
- Seizures or fainting
- Difficulty with walking, climbing stairs, or lifting objects
- Drooping eyelids, dry eyes, or blurred vision
- Swallowing difficulties
- Dizziness upon standing
- Dry mouth
- Constipation
- Erectile dysfunction
- Temporary improvement in strength during exercise, followed by reduction as exercise continues
If you experience any of these symptoms, it’s important to discuss them with a medical professional to determine the underlying cause and appropriate treatment plan.
Stages of Lung Cancer

The stage of lung cancer refers to its size and whether it has spread to other parts of the body, and plays a crucial role in determining the appropriate treatment approach.
Diagnostic tests and scans provide information about the stage of cancer, although it may not always be possible to determine the stage with certainty until after surgery.
Doctors typically use the TNM system (Tumour, Node, Metastasis) to stage lung cancer, which is the most commonly used staging method.
For small cell lung cancer, there is a simplified staging system called limited and extensive stage, although it is less frequently used as more is learned about this type of cancer.
In the number staging system, lung cancer are categorized based on the size and spread of the tumor, as well as the extent of the cancer’s reach within the body.
These stages include:
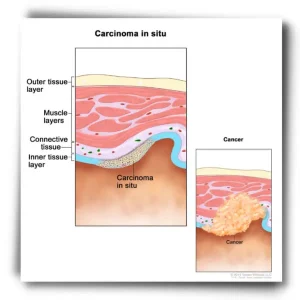
- Stage 0 (in-situ): The cancer is located in the top lining of the lung or bronchus and has not spread to other parts of the lung or outside of the lung.
- Stage I: The cancer is confined to the lung and has not spread beyond it.
- Stage II: The cancer is larger than in Stage I, has spread to nearby lymph nodes within the lung, or there are multiple tumors in the same lobe of the lung.
- Stage III: The cancer is larger than in Stage II, has spread to nearby lymph nodes or structures, or there are multiple tumors in a different lobe of the same lung.
- Stage IV: The cancer has spread to the other lung, the fluid surrounding the lung, the fluid surrounding the heart, or distant organs.
Knowing the stage of lung cancer is vital in determining the best course of treatment for each patient.
Types of Lung Cancer

The type of lung cancer is determined by the type of cell in which it originated. Identifying the type of lung cancer helps doctors determine the most appropriate treatment approach.
Primary lung cancer originates in the lung, whereas secondary lung cancer spreads to the lungs from another part of the body.
There are two main types of primary lung cancer:
Small cell lung cancer (SCLC): This type of neuroendocrine tumor develops in the neuroendocrine cells of the lung. It is typically caused by smoking and tends to spread quickly.
Non-small cell lung cancer (NSCLC): This type is more common than SCLC and accounts for around 80-85% of all lung cancers. It is further divided into three subtypes:
- Adenocarcinoma: The most common subtype, it develops in the mucus-producing gland cells lining the airways.
- Squamous cell carcinoma: This type develops in the flat cells covering the airways and is more likely to grow near the center of the lung.
- Large cell carcinoma: The cells appear larger than a typical cell under a microscope.
Less common types of NSCLC include adenosquamous carcinoma and sarcomatoid carcinoma.
If the cancer cells appear undeveloped under the microscope, additional tests may be required to determine the type of cancer.
Other rare types of lung tumors include
- Salivary gland-type tumors
- Lung sarcoma
- Lung lymphoma
Treatment for these types of cancers differs from SCLC and NSCLC.
Risks and causes of lung cancer

A risk factor is anything that increases the likelihood of developing a particular disease. Different cancers have different risk factors, and having one or more of these risk factors does not necessarily mean that one will develop cancer.
- The primary risk factor for lung cancer is smoking tobacco. Approximately 7 out of 10 lung cancers are caused by smoking, including exposure to secondhand smoke.
Even light or occasional smoking can increase the risk of lung cancer, with the risk increasing the longer and more frequently one smokes.
Quitting smoking is the best way to reduce the risk of developing lung cancer.
- Exposure to certain substances in the workplace, such as asbestos, silica, and diesel exhaust, can also increase the risk of lung cancer.
- Outdoor air pollution is another risk factor, with exposure causing about 1 in 10 cases of lung cancer in the UK.
- Previous lung diseases, such as COPD and idiopathic pulmonary fibrosis, can also increase the risk of developing lung cancer.
- Exposure to radon gas, a naturally occurring radioactive gas that can build up in homes and other buildings, can increase the risk of lung cancer, especially in smokers.
- Having a family history of lung cancer or taking high doses of beta-carotene supplements may also increase the risk.
While there are ongoing studies on how genetics affect the risk of developing lung cancer, many stories about other potential causes lack evidence or clarity.
How to detect Lung Cancer?
Imaging tests: X-rays, CT scans, and MRI scans can be used to detect any abnormal growths or nodules in the lungs.
Sputum cytology: This test involves examining a sample of mucus coughed up from the lungs to check for cancer cells.
Biopsy: A tissue sample is taken from the lung and examined under a microscope to determine if it is cancerous.
Bronchoscopy: A thin, flexible tube with a camera is inserted into the lungs to examine the airways and take a tissue sample if needed.
PET scan: A PET scan uses a special dye to show areas of abnormal activity in the body, which can help detect cancerous cells.
It is important to note that early-stage lung cancer may not have any noticeable symptoms, which is why routine screenings are recommended for individuals at high risk, such as current or former smokers.
These screenings typically involve a low-dose CT scan. It is best to discuss your personal risk factors and screening options with your healthcare provider.
How is it treated?

The treatment approach for lung cancer is determined by various factors, such as
- The type of lung cancer (non-small-cell or small-cell mutations on cancer)
- The size and location of the tumor
- The stage of cancer
- The patient’s overall health
It can be challenging to choose the most suitable treatment option. Although your cancer team may offer recommendations, ultimately, the final decision rests with you.
Common treatments for lung cancer include:
Surgery: If the cancer is confined to the lung and has not spread, surgery may be an option to remove the tumor and any affected lymph nodes.
Radiation therapy: This treatment uses high-energy radiation to kill cancer cells. It may be used alone or in combination with other treatments.
Chemotherapy: Chemotherapy involves using drugs to kill cancer cells. It can be administered orally or intravenously.
Targeted therapy: Targeted therapy uses drugs that specifically target cancer cells that have certain genetic mutations.
Immunotherapy: Immunotherapy works by boosting the body’s immune system to help it fight cancer.
Palliative care: Palliative care is a form of care that is focused on relieving symptoms and improving quality of life for patients with advanced or incurable lung cancer.
Treatment plans are individualized to the patient and may involve a combination of these therapies. It’s important to discuss treatment options with your healthcare provider to determine the best course of action for you.
In a Nutshell
In conclusion, the passing of beloved comedian Sean Lock left fans wondering about the type of cancer he had.
Through this article, we tried to delve into the details of his diagnosis and battle with lung cancer, shedding light on the various symptoms and risk factors associated with this disease.
It is important to raise awareness about the potential dangers of lung cancer and to encourage individuals to seek medical attention if they notice any of the common symptoms.
We hope that this article has provided valuable information and insight into the topic of lung cancer and its impact on individuals and their loved ones.
Take Care of Yourself!