“संकेतों को जानें: ब्लड कैंसर का जल्द पता लगने से आपकी जान बच सकती है।”
रक्त कैंसर वास्तव में क्या है?
ब्लड कैंसर एक प्रकार का कैंसर है जो रक्त, अस्थि मज्जा और लसीका तंत्र की कोशिकाओं को प्रभावित करता है। यह एक गंभीर और संभावित जीवन-धमकाने वाली स्थिति है जो विभिन्न प्रकार के लक्षण पैदा कर सकती है।
ब्लड कैंसर के सामान्य लक्षणों में थकान, बुखार, रात को पसीना आना, वजन कम होना, हड्डियों में दर्द और लिम्फ नोड्स में सूजन शामिल हैं। अन्य लक्षणों में रक्ताल्पता, आसान खरोंच या रक्तस्राव, और बार-बार संक्रमण शामिल हो सकते हैं।
विभिन्न प्रकार के रक्त कैंसर
रक्त कैंसर के कई अलग-अलग प्रकार हैं, प्रत्येक के अपने विशिष्ट लक्षण और उपचार हैं।
रक्त कैंसर का सबसे आम प्रकार ल्यूकेमिया है। ल्यूकेमिया सफेद रक्त कोशिकाओं का कैंसर है, जो संक्रमण से लड़ने के लिए जिम्मेदार हैं।
ल्यूकेमिया के 4 मुख्य प्रकार हैं, वे हैं:
एक्यूट लिम्फोब्लास्टिक ल्यूकेमिया (ALL)
तेजी से बढ़ने वाला कैंसर जो सफेद रक्त कोशिकाओं को प्रभावित करता है
उदाहरण: बी-सेल ऑल, टी-सेल ऑलतीव्र माइलॉयड ल्यूकेमिया (एएमएल)
- तेजी से बढ़ने वाला कैंसर जो श्वेत रक्त कोशिकाओं, लाल रक्त कोशिकाओं और प्लेटलेट्स को प्रभावित करता है
- उदाहरण: M0 AML, M1 AML, M2 AML
क्रोनिक लिम्फोसाइटिक ल्यूकेमिया (सीएलएल)
- धीमी गति से बढ़ने वाला कैंसर जो श्वेत रक्त कोशिकाओं को प्रभावित करता है
- उदाहरण: बी-सेल सीएलएल, टी-सेल सीएलएल
क्रोनिक माइलॉयड ल्यूकेमिया (CML)
- धीमी गति से बढ़ने वाला कैंसर जो श्वेत रक्त कोशिकाओं को प्रभावित करता है
- उदाहरण: बीसीआर-एबीएल पॉजिटिव सीएमएल, एबीएल1 पॉजिटिव सीएमएल।
एक अन्य प्रकार का रक्त कैंसर लिम्फोमा है। लिंफोमा लसीका प्रणाली का एक कैंसर है, जो प्रतिरक्षा प्रणाली का हिस्सा है।
यह दो मुख्य प्रकारों में बांटा गया है: हॉजकिन का लिंफोमा और गैर-हॉजकिन का लिंफोमा।
लिंफोमा के लक्षणों में लिम्फ नोड्स में सूजन, बुखार, रात को पसीना आना और वजन घटना शामिल हो सकते हैं। लिंफोमा के उपचार में आमतौर पर कीमोथेरेपी, विकिरण, और/या स्टेम सेल प्रत्यारोपण शामिल होता है।
मायलोमा एक प्रकार का कैंसर है जो अस्थि मज्जा में प्लाज्मा कोशिकाओं को प्रभावित करता है।मायलोमा के लक्षणों में हड्डी में दर्द, थकान और एनीमिया शामिल हो सकते हैं। मायलोमा के उपचार में आमतौर पर कीमोथेरेपी, विकिरण और/या स्टेम सेल प्रत्यारोपण शामिल होता है।
अंत में, मल्टीपल मायलोमा होता है, जो एक प्रकार का कैंसर है जो शरीर के कई क्षेत्रों को प्रभावित करता है। मल्टीपल मायलोमा के लक्षणों में हड्डी में दर्द, थकान और एनीमिया शामिल हो सकते हैं। मल्टीपल मायलोमा के उपचार में आमतौर पर कीमोथेरेपी, विकिरण और/या स्टेम सेल प्रत्यारोपण शामिल होता है।
रक्त कैंसर एक भयावह और भारी निदान हो सकता है, लेकिन यह याद रखना महत्वपूर्ण है कि उपचार उपलब्ध हैं और रक्त कैंसर वाले कई लोग पूर्ण और उत्पादक जीवन जी सकते हैं। सही उपचार और सहायता से, लक्षणों को प्रबंधित करना और एक लंबा और स्वस्थ जीवन जीना संभव है।
रक्त कैंसर के चरण
आमतौर पर रक्त कैंसर के 4 चरण होते हैं जैसे ल्यूकेमिया, लिम्फोमा और मल्टीपल मायलोमा:
- स्टेज 1: कैंसर स्थानीय होता है और शरीर के केवल एक छोटे से हिस्से को प्रभावित करता है।
- चरण 2: कैंसर आस-पास के ऊतकों में फैल गया है।
- चरण 3: कैंसर शरीर के अन्य भागों में फैल गया है और अधिक व्यापक है।
- स्टेज 4: कैंसर शरीर के दूर के हिस्सों में फैल गया है और इसे उन्नत या मेटास्टैटिक माना जाता है।
रक्त कैंसर के लिए उपचार के विकल्प

रक्त कैंसर (हेमेटोलॉजिकल कैंसर के रूप में भी जाना जाता है) के उपचार के विकल्पों में शामिल हैं:
कीमोथेरेपी (chemotherapy): एक प्रकार का उपचार जो कैंसर कोशिकाओं को नष्ट करने के लिए दवाओं का उपयोग करता है। कीमोथेरेपी दवाओं के उदाहरणों में साइटोक्सन, मेथोट्रेक्सेट और एड्रैमाइसिन शामिल हैं।
विकिरण चिकित्सा (Radiation therapy ): एक उपचार जो कैंसर कोशिकाओं को नष्ट करने के लिए उच्च-ऊर्जा विकिरण का उपयोग करता है। इस थेरेपी का उपयोग आमतौर पर स्थानीय रक्त कैंसर जैसे हॉजकिन के लिंफोमा या कुछ प्रकार के ल्यूकेमिया के इलाज के लिए किया जाता है।
स्टेम सेल प्रत्यारोपण: एक उपचार जो रोगग्रस्त रक्त बनाने वाली कोशिकाओं को स्वस्थ कोशिकाओं से बदल देता है। यह प्रक्रिया आमतौर पर उच्च खुराक कीमोथेरेपी या विकिरण चिकित्सा के बाद की जाती है।
लक्षित चिकित्सा: एक प्रकार का उपचार जो कैंसर कोशिकाओं के विकास और प्रसार में शामिल विशिष्ट अणुओं को लक्षित करने के लिए दवाओं का उपयोग करता है। लक्षित उपचारों के उदाहरणों में ग्लीवेक और इम्ब्रूविका शामिल हैं।
इम्यूनोथेरेपी: एक प्रकार का उपचार जो प्रतिरक्षा प्रणाली को कैंसर कोशिकाओं से लड़ने में मदद करता है। इम्युनोथैरेपी के उदाहरणों में सीएआर टी-सेल थेरेपी और चेकपॉइंट इनहिबिटर शामिल हैं।
उपचार का विकल्प कई कारकों पर निर्भर करेगा, जिसमें रक्त कैंसर का प्रकार और चरण, रोगी का समग्र स्वास्थ्य और उपचार के संभावित दुष्प्रभाव शामिल हैं।
ऑन्कोलॉजिस्ट, हेमेटोलॉजिस्ट और अन्य विशेषज्ञों सहित चिकित्सा पेशेवरों की एक टीम प्रत्येक रोगी के लिए एक व्यक्तिगत उपचार योजना विकसित करने के लिए मिलकर काम करेगी।
इसका उपयोग अक्सर रक्त कैंसर जैसे क्रोनिक माइलॉयड ल्यूकेमिया और मल्टीपल मायलोमा के इलाज के लिए किया जाता है।
कोई फर्क नहीं पड़ता कि आपको किस प्रकार का रक्त कैंसर है, उपचार के विकल्प उपलब्ध हैं। सही उपचार योजना के साथ, आप अपनी स्थिति का प्रबंधन कर सकते हैं और यहां तक कि छूट भी प्राप्त कर सकते हैं।
अपने उपचार विकल्पों के बारे में अपने डॉक्टर से बात करना और जो आपके लिए सही है उसे ढूंढना महत्वपूर्ण है। सही देखभाल और सहयोग से आप लंबा और स्वस्थ जीवन जी सकते हैं।
ब्लड कैंसर के शुरुआती चेतावनी के संकेत
रक्त कैंसर के प्रारंभिक चेतावनी संकेत कैंसर के प्रकार के आधार पर भिन्न हो सकते हैं, लेकिन कुछ सामान्य संकेतों में शामिल हैं:
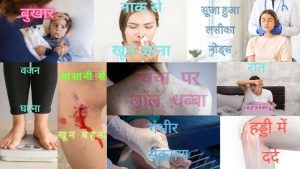
• अस्पष्टीकृत थकान: रात को अच्छी नींद लेने के बाद भी हर समय थकान महसूस होना ब्लड कैंसर का संकेत हो सकता है।
• बिना वजह वजन कम होना: बिना कोशिश किए वजन कम होना कैंसर का संकेत हो सकता है।
• बुखार: बुखार जो दूर नहीं हो रहा है वह संक्रमण का संकेत हो सकता है, जो रक्त कैंसर का संकेत हो सकता है।
• रात को पसीना आना: रात में बिना किसी स्पष्ट कारण के पसीना आना ब्लड कैंसर का संकेत हो सकता है।
• सूजन लिम्फ नोड्स: गर्दन, बगल या ग्रोइन में लिम्फ नोड्स में सूजन ब्लड कैंसर का संकेत हो सकता है।
• चोट लगना या आसानी से खून बहना: अस्पष्ट चोट या खून बहना ब्लड कैंसर का संकेत हो सकता है।
• सांस फूलना: सांस फूलना एनीमिया का संकेत हो सकता है, जो ब्लड कैंसर का संकेत हो सकता है।
यदि आप इनमें से किसी भी लक्षण का अनुभव करते हैं, तो तुरंत अपने डॉक्टर से बात करना महत्वपूर्ण है। रक्त कैंसर के प्रबंधन के लिए प्रारंभिक पहचान और उपचार महत्वपूर्ण हैं। सही देखभाल और सहयोग से आप लंबा और स्वस्थ जीवन जी सकते हैं।
रक्त कैंसर का निदान कैसे करें?

रक्त कैंसर का निदान करना एक जटिल प्रक्रिया हो सकती है, क्योंकि कई प्रकार के रक्त कैंसर होते हैं और प्रत्येक प्रकार के लक्षणों का अपना सेट होता है।
रक्त कैंसर के निदान में पहला कदम एक शारीरिक परीक्षा है और अपने चिकित्सक के साथ अपने चिकित्सकीय इतिहास पर चर्चा करना है। स्थिति का निदान करने में मदद के लिए आपका डॉक्टर रक्त परीक्षण, इमेजिंग परीक्षण और बायोप्सी का भी आदेश दे सकता है।
रक्त परीक्षण सफेद रक्त कोशिकाओं, लाल रक्त कोशिकाओं और प्लेटलेट्स के असामान्य स्तर का पता लगाने में मदद कर सकता है। एक्स-रे, सीटी स्कैन और एमआरआई जैसे इमेजिंग टेस्ट ट्यूमर या अन्य की पहचान करने में मदद कर सकते हैं।
बायोप्सी एक ऐसी प्रक्रिया है जिसमें शरीर से ऊतक का एक नमूना लिया जाता है और कैंसर कोशिकाओं की तलाश के लिए माइक्रोस्कोप के तहत जांच की जाती है।
यद्यपि रक्त कैंसर का निदान एक जटिल प्रक्रिया हो सकती है, यह याद रखना महत्वपूर्ण है कि प्रारंभिक निदान और उपचार परिणाम में बड़ा अंतर ला सकता है।
यदि आपके पास कोई लक्षण है जो रक्त कैंसर से संबंधित हो सकता है, तो जितनी जल्दी हो सके अपने डॉक्टर से बात करना महत्वपूर्ण है। सही उपचार और सहायता से, आप अपनी स्थिति का प्रबंधन कर सकते हैं और पूर्ण और स्वस्थ जीवन जी सकते हैं।
ब्लड कैंसर से जुड़ी अधिक या किसी अन्य जानकारी के लिए यहां क्लिक करें।